UNDERCOVER INVESTIGATION EXPOSES CRUEL AND UNNECESSARY RABBIT PYROGEN TESTING
Release 7/10/2025 - An undercover investigation by LCA exposes the cruelty of rabbit pyrogen tests (RPT's) at a North American toxicology research facility. Pyrogens are fever-causing substances that can be found in parenteral pharmaceuticals (any drugs administered through a route other than the digestive tract). RPT's are used to ensure medications are pyrogen-free. During pyrogen testing, rabbits are forced into restraints, have test items injected into their ear veins, and are monitored with a temperature probe to determine if they develop a fever. U.S. RESIDENTS: TAKE ACTION
WATCH AND SHARE THE UNDERCOVER VIDEO
THE NIGHTMARE LIVES OF RABBITS USED IN PYROGEN TESTING
Rabbit pyrogen testing is inherently cruel. Purpose-bred rabbits are used in the studies and live their entire lives in cages at research facilities. They are only taken out of their cages for the pyrogen test or other procedures, like ear tagging or having their weight taken. All their natural instinctive behaviors, like digging, running, jumping and socializing, have been thwarted. When the rabbits can no longer be used for testing, they are killed. At this facility, the rabbits were killed via an injection of pentobarbital into an ear vein and then necropsied.
The investigation documented:
- Rabbits struggling as they are put into restraint devices.
- Rabbits kicking in the restrainers, putting them at risk of broken backs.
- Repeated ear injections causing rabbit ears to become scarred, bloody, and hardened. The damaged ear makes it difficult for technicians to find healthy ear veins for injections, and subsequently, the rabbits are jabbed multiple times with needles.
- A technician struggling for over ten minutes to find an ear vein to inject pentobarbital (the killing agent) into a rabbit.
- Rabbits being killed and necropsied in full view of other rabbits.
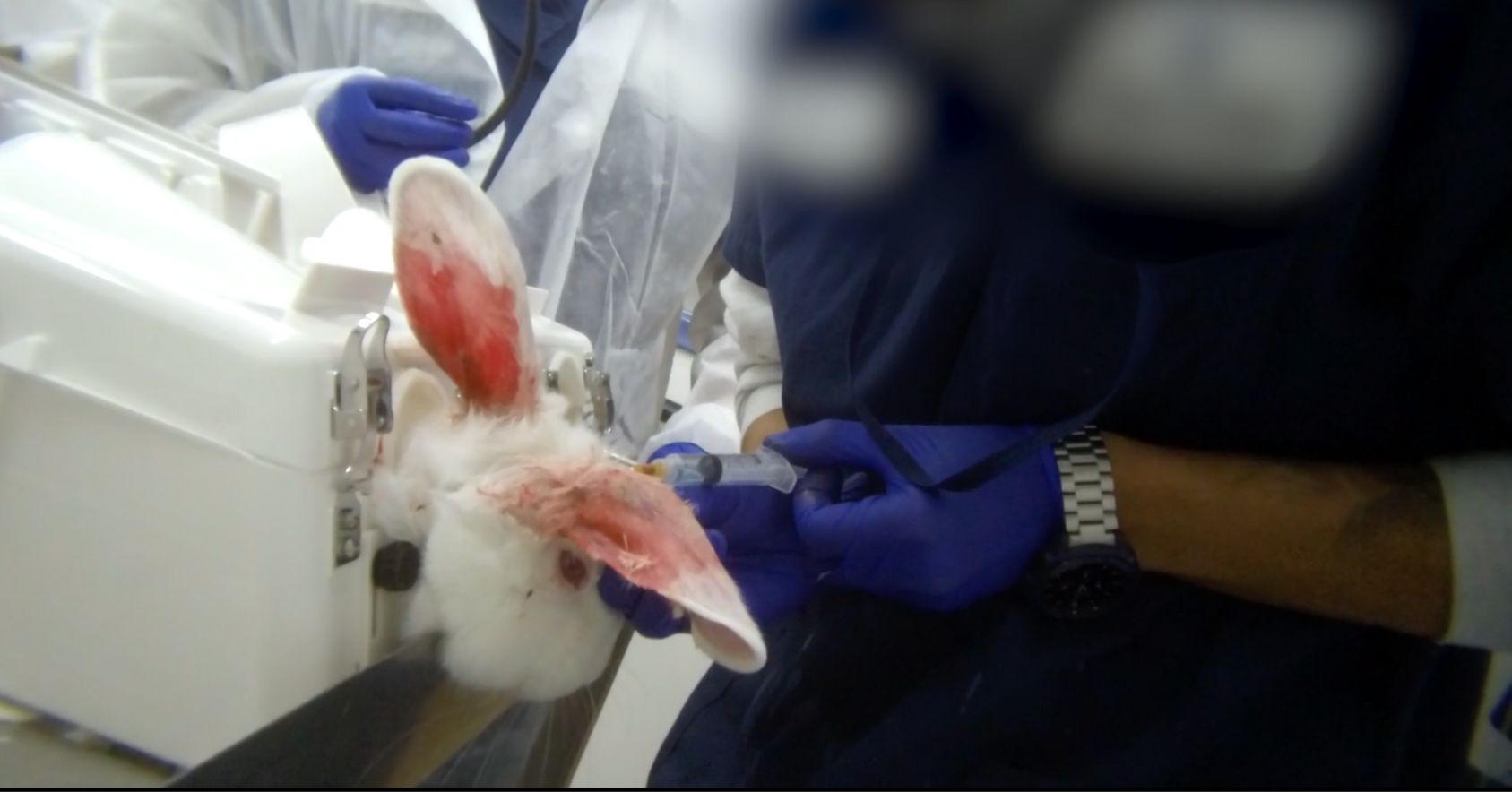 Injection with bloody ears |
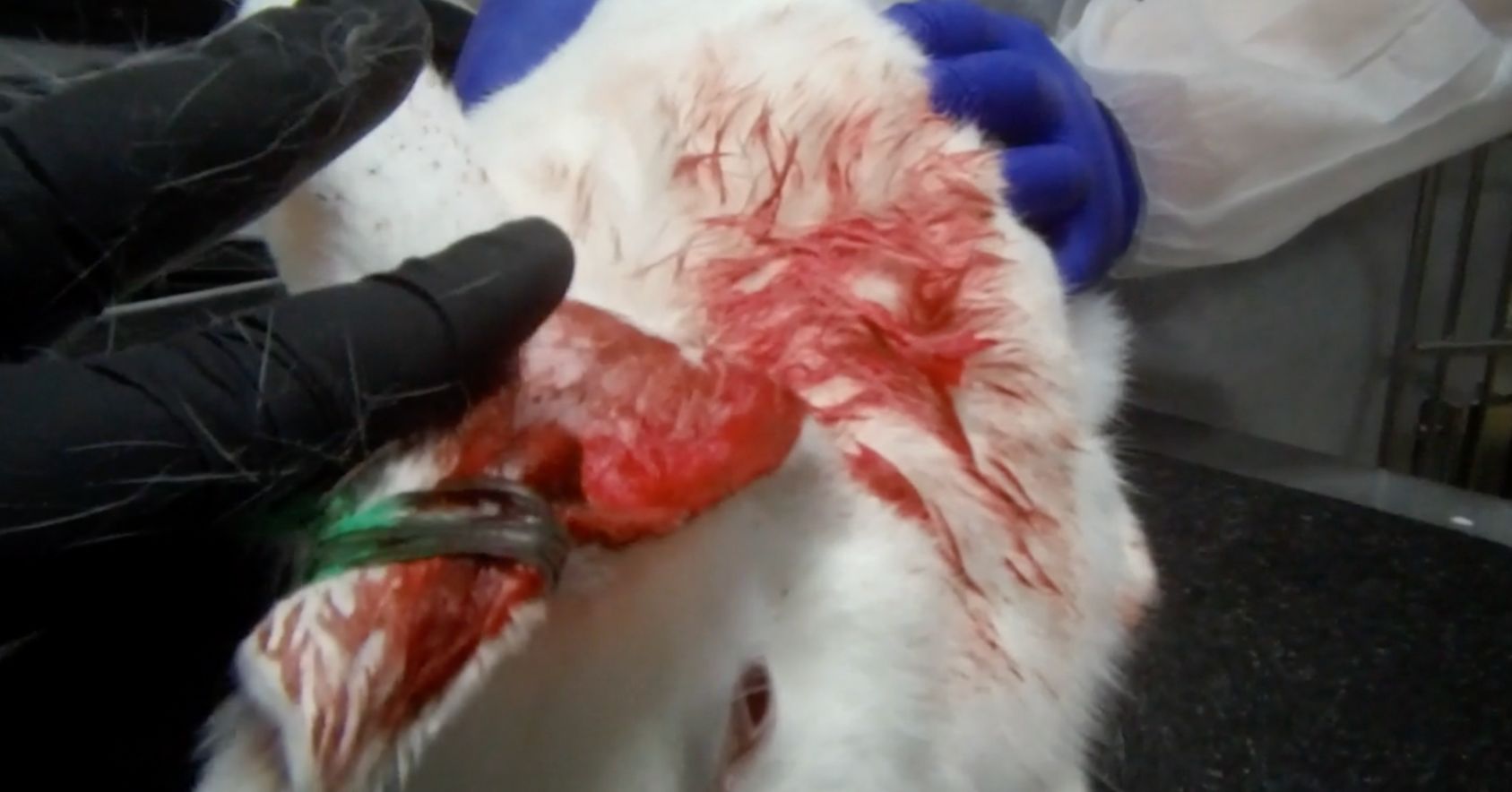 Bloody ear closeup |
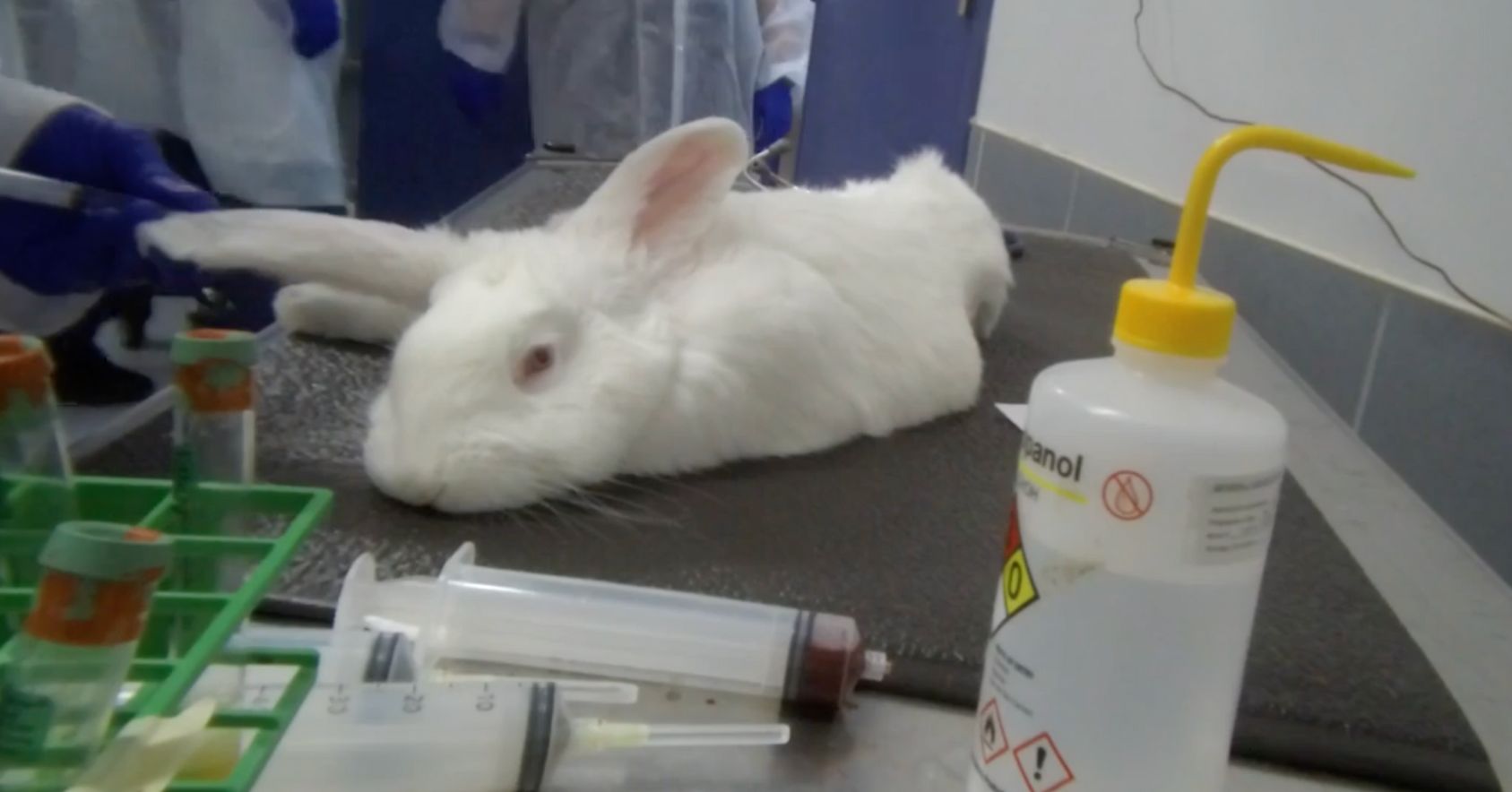 Dead rabbit |
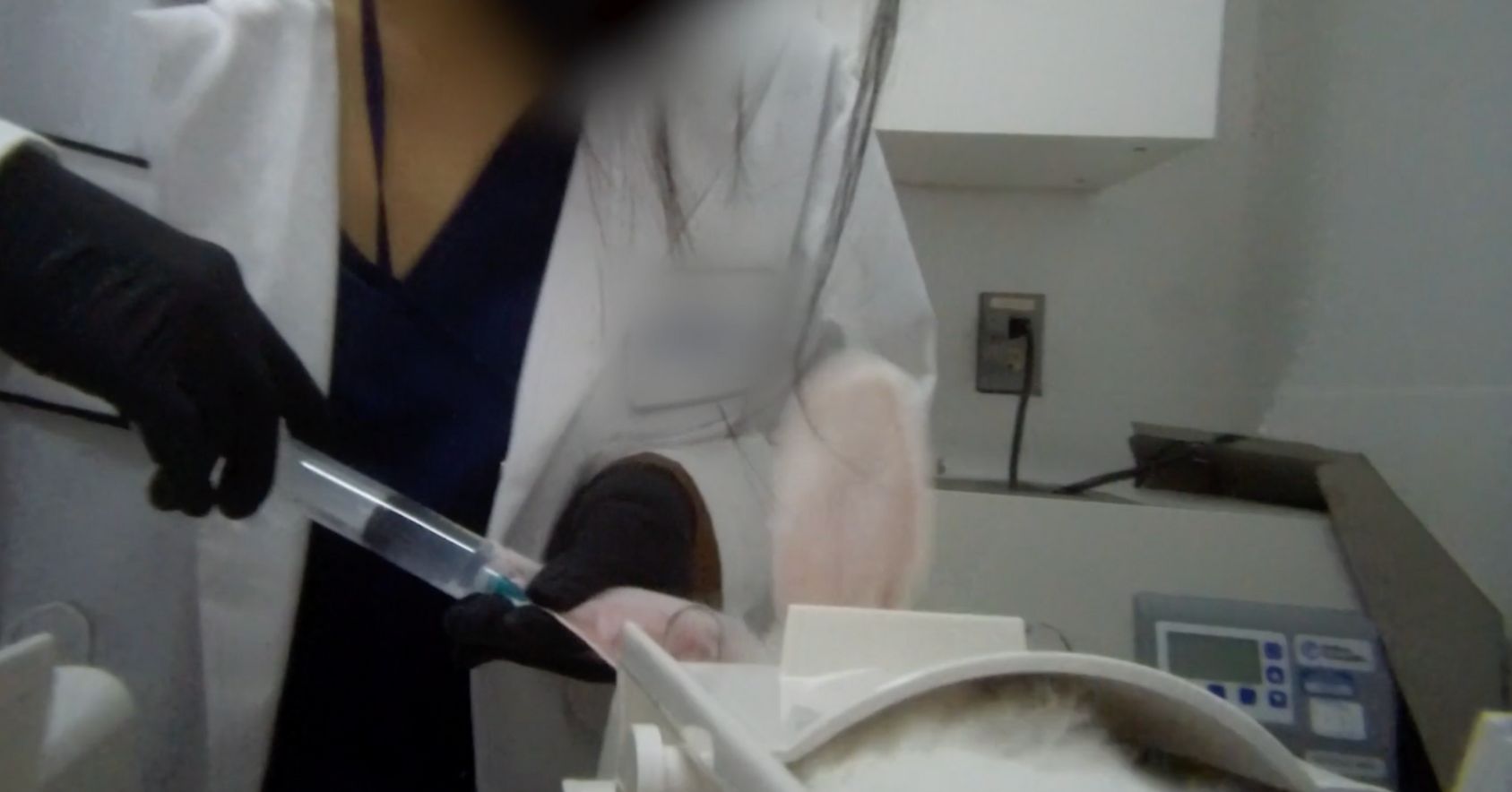 Ear vein injection |
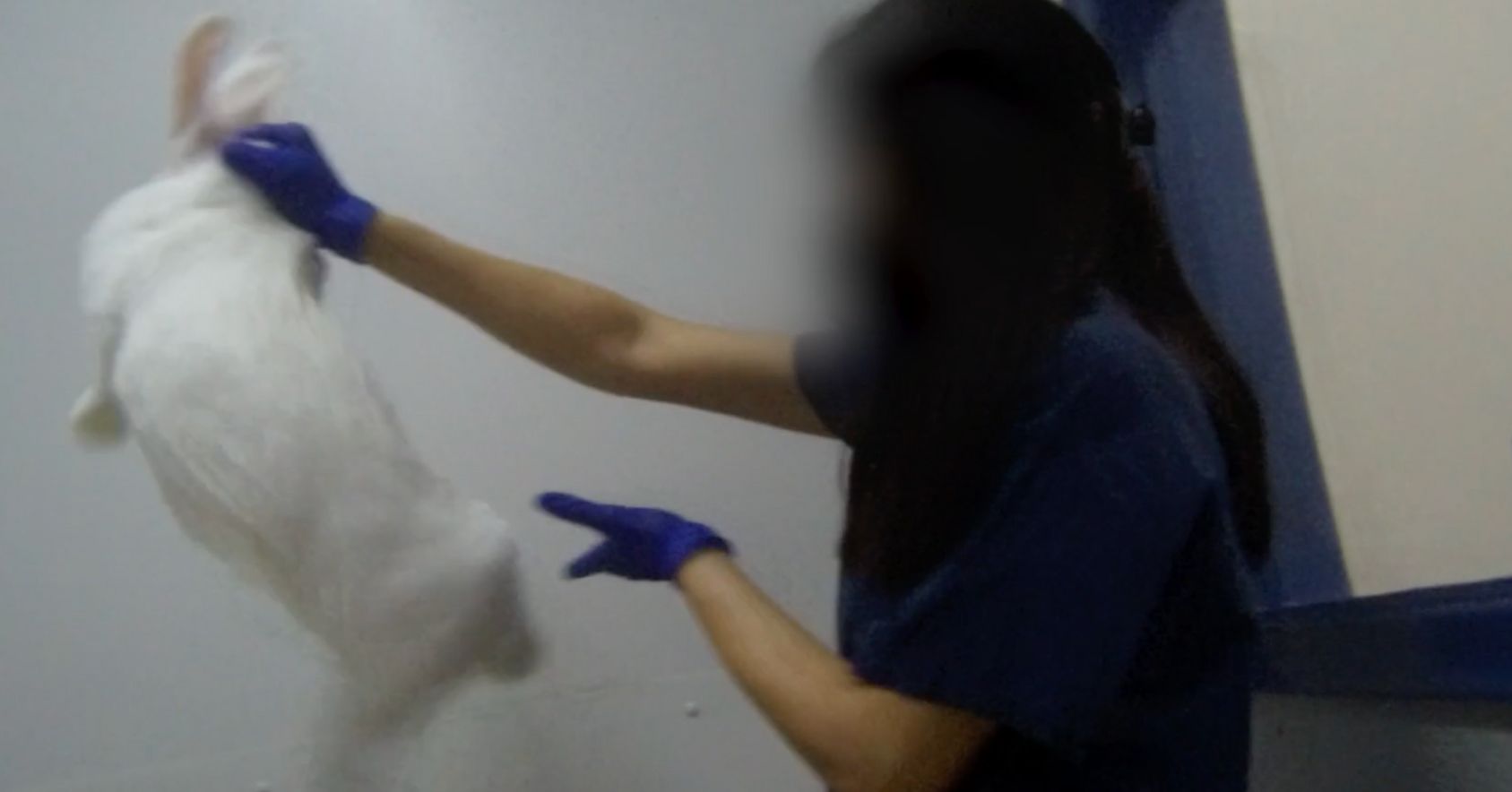 Rabbit struggling |
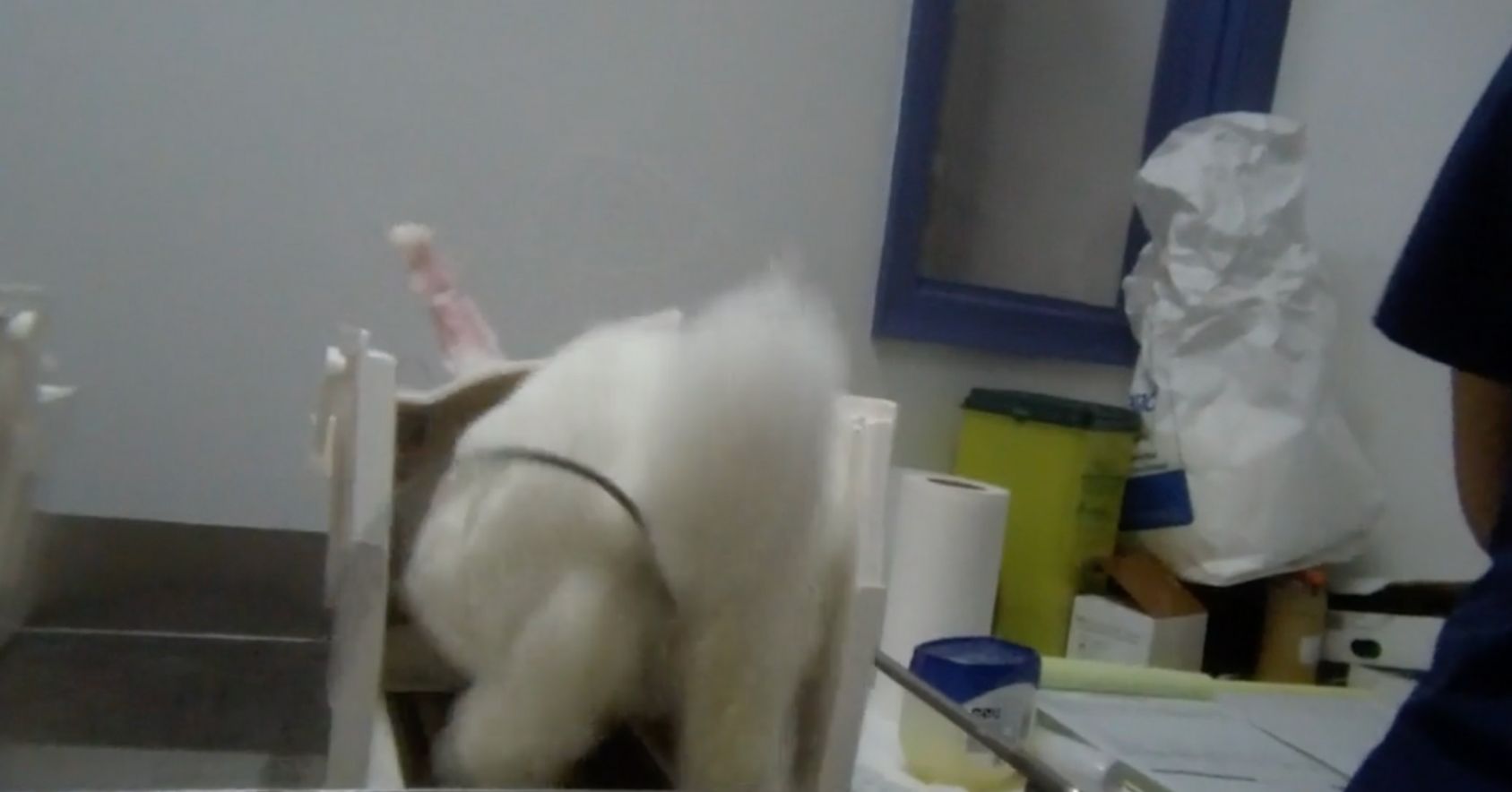 Kicking in restrainer |
 Restraint device |
 Restraint devices |
RABBIT PYROGEN TESTING IS UNNECESSARY
It is estimated that approximately 400,000 rabbits are used worldwide in RPT's each year. Rabbits have been favored in pyrogen tests because scientists believe their bodies react to pyrogens in a way that is similar to humans. It is common practice to use three rabbits to test a single medicinal product. Purpose-bred rabbits are used for pyrogen studies. New Zealand Whites, sold to research companies by vendors such as Charles River and Inotiv, are a common breed in laboratories.

Ad for a research rabbit from the Inotiv website
Due to the possible presence of pyrogens in any injectable medications, pyrogen testing is mandated by the National Institute of Health (NIH). However, it is not mandatory to use rabbits for pyrogen testing because non-animal alternatives are available. The Monocyte Activation Test (MAT) is an example of a reliable, non-animal alternative to rabbit pyrogen testing.
EUROPEAN PHARMACOPOEIA STOPS RABBIT PYROGEN TESTING
In June 2024, the European Pharmacopoeia, which is responsible for the quality control of medications in Europe, announced it would stop rabbit pyrogen testing by July 1, 2025. Medicine manufacturers would be responsible for selecting non-animal-based methods for pyrogen testing that are traditionally done on rabbits (such as the MAT). A press release issued by the European Pharmacopoeia in July 2024 states in part, "...the EPC is committed to the reduction of animal usage wherever possible in its pharmacopeial testing. This historic step is an illustration of this commitment and will have a significant impact on the replacement, reduction, and refinement of animal tests..."
THE UNITED STATES CONTINUES THE ABUSE
In the United States, despite non-animal alternatives to RPT's being readily available, there is no law requiring pharmaceutical companies, contract toxicology facilities, or other government institutions to move away from animal testing methods.
In December 2022, Congress passed the FDA Modernization Act 2.0. The Act ended the requirement that experimental drugs be tested on animals, paving the way for non-animal testing. In April 2025, the Food and Drug Administration (FDA) released a roadmap to phase out animal testing for drug development.
Starting in 2025, and over the next 3-5 years, the FDA aims to drastically reduce animal testing, making it the "exception rather than the norm". The FDA plans to work with other U.S. government agencies, including the NIH and the Department of Veterans Affairs, to promote alternative methods.
It is a positive step that federal laws have been passed to move away from animal testing; however, no law requires it. There have been laws passed at the state levels in CA, NY, NJ, and VA to mandate the use of non-animal testing methods in certain scenarios, but no federal laws.
It is unclear why pharmaceutical companies and other institutions choose to partake in unnecessary animal testing when there are viable alternatives available. Heavy lobbying by companies that have financial stakes in animal testing likely plays a major role. Other key factors might include skepticism or lack of knowledge about the acceptability of non-animal methods; unwillingness to change; and unreliable bureaucratic approval.
HOW YOU CAN HELP
U.S. RESIDENTS: Click here to contact your elected officials to urge them to stop cruel and needless animal testing and support non-animal testing alternatives.
Don't live in the U.S.? You can still help! Sign LCA's petition against animal testing!














